Since the first soaps derived from animal fats and alkalis were sold in the 1700s, laundry detergents have gone long. In the 1950s, laundry detergents were introduced to the market, giving housewives greater fabric-care options. However, the most major advance in laundry occurred in the 1970s. In this guide, we will talk about laundry detergent chemical formula.
What are Detergents?

Detergent is a soap-free cleaning agent that cleans the substance in solution using a surfactant ingredient.
Soap-free soaps are a term used to describe detergents. They are effective even in hard or saltwater, unlike soap, because they do not create scum. Cleaners are better because they do not form insoluble calcium and magnesium salts with hard water.
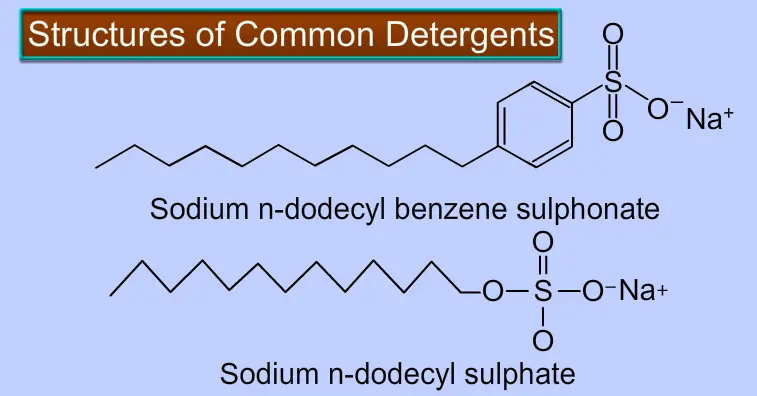
The detergent is a sodium salt of long-chain benzene sulfonic acid or sodium salt of long-chain alkyl hydrogen sulfate, both of which have water-cleaning capabilities. Like soaps, they contain anionic groups such as sulfonate groups or sulfate groups and a long-chain hydrocarbon, a nonionic group. They are also called soap-free soaps, detergents, Syndets, or soap.
Type of Laundry Detergents

The detergent molecule comprises an oil-soluble hydrocarbon and a water-soluble polar group. Detergents are divided into three categories.
Detergent with anionic ions
An anionic hydrophilic group is included in anionic detergents, which helps clean. These are long-chain sulfonated hydrocarbons or alcohol sodium salts.
Detergent catalytic
Cationic detergents are quaternary ammonium compounds like chlorides, bromides, and acetates.
Some of these detergents are used as antiseptics due to their germicidal capabilities. Conditioners and hair conditioners also contain cationic detergents.
Non-ionic detergent
Nonionic detergents have a neutral group in their molecule as a surfactant group that can establish hydrogen bonds with water. Monoesters of polyhydric alcohols or polyether produced from ethylene oxide are nonionic detergents. Nonionic detergents include pentaerythritol stearate and polyether.
Uses of Detergents

- Powdered and liquid detergents can be used for purposes other than cleaning clothes or dishes.
- They are mainly used as cleaning agents in laundry and dishes.
- Tiles, floors, worktops, bathtubs, and toilets can be cleaned with any type of detergent.
- Powder detergent can soak up oil spilled on the garage floor or the street.
- Detergents increase the softness of water.
- Sprinkle detergent powder on any moss that grows in cracks on your stairs, sidewalks, or driveways. Let it brown for a few days before brushing it out of the gaps.
- They are also used to solubilize and crystallize membrane proteins and prevent nonspecific binding.
- Detergents are used for cell permeabilization, lysis, gel electrophoresis, and other laboratory procedures.
Laundry Detergent Chemical Formula
The detergent is a type of emulsifier known as sodium dodecylbenzene sulfonate. Its chemical formula is sodium dodecylbenzene sulfonate sodium (C18H29NaO3S). Soap has the chemical formula C17H35COONa or sodium stearate, whereas detergent has C18H29NaO3S. In both hard and soft water, a synthetic detergent is a non-soap synthetic substance that effectively cleanses and operates similarly to a surfactant.
- Surfactants are typically found in detergents.
- Detergents work because of their amphipathic nature, which means one side of the chemical is hydrophobic and repels water. In contrast, the other side of the compound is hydrophilic and easily binds to water.
This chemical compound has excellent foaming properties and can be used with a wide range of additional additives. Detergents work because they have an amphipathic structure, indicating one side of the chemical is hydrophobic and repels water while the other is hydrophilic and readily binds to it.
The hydrophobic region of the detergent will react easily and attract other molecules present in its environment; thus, it accumulates on the detergent while the hydrophilic region of the detergent ensures that the whole compound, as well as the attracted particles, is easily washed off with water.
Detergents can be used for various cleaning purposes due to the amphipathic structure of detergent molecules. Degreasing the skin is also recommended as hydrophobic areas will easily attract fat and other fat molecules, which consist mainly of carbon tails.
Although they are similar to soap, detergents are quite different. Soaps are made from natural resources like fats and oils, while detergents are synthetic. In addition, detergents contain sodium salts.
How to Choose the Best Detergent

In the laundry detergent stores, there are a lot of options. What criteria do you use to make your decision? The best option is one that fulfills your family’s needs for efficiency on various floors, as well as odor, shape (powder, liquid, or single dosage), and price preferences.
Here’s what you need to know to get started. Examine your family’s laundry, paying particular attention to the varieties of stains and the amount of grime on the clothing. If most of your garments are only lightly soiled and have a few spots, a less expensive detergent and a decent stain remover may suffice.
Read laundry detergent labels or go online to read the ingredients. It is important to investigate surfactants and enzymes to remove dirt and stains. Cheap brands have fewer of these components and will not clean them well.
You may find that the two formulas on the laundry rack will meet your needs; one detergent for lightly soiled clothes and one for heavily soiled clothes. Although most detergents work in cold water, it is best to choose one formulated for cold water if you intend to use only cold water.
Concentrated and ultra-formulations are now available in liquids and powders. Although packaged in smaller sizes, they offer the same cleaning power as their larger non-concentrated counterparts.
To determine the correct amount to use, follow the instructions on the label and use the supplied cup or measuring spoon. These products simply remove water or additional costs, making them easier and cheaper to ship and store.
Single-dose packs and capsules are still concentrated and can save you money by avoiding overuse.
Many people choose their lingerie based on their scent. Make sure the dirt is removed, not just covered with perfume.
Frequently Asked Questions
What is the formula for detergent chemicals?
Soap has the chemical formula C17H35COONa or sodium stearate, whereas detergent has C18H29NaO3S. A synthetic detergent is a non-soap synthetic material that is an effective cleanser and functions similarly to a surfactant in both hard and soft water.
What is the most important element in washing detergent?
Most laundry detergents contain alkalis, soluble salts with an acid-reactive base to neutralize them. They remove dirt and stains from textiles without causing extreme friction. Potassium and sodium are soluble alkali metal salts that work well as degreasers.
Did soap and detergent have different chemical compositions?
Detergents are ammonium or sulfur salts of long-chain carboxylic acids, whereas soaps are sodium (Na) or potassium (K) salts of specific long-chain carboxylic acids (COOH).
How can you explain the detergent solution?
A dilute solution is a surfactant or a combination of surfactants having cleaning capabilities. The detergent has a general R-SO4-, Na + structure, where R is a long chain alkyl group comparable to soap.
What is the mixture of detergent solutions?
Use 1.25 dry ounces of powder per gallon of water or 10 grams per liter to make a weighted solution. Alternatively, you can add 1-ounce tablespoon (a large teaspoon is usually fine) to 1 gallon of water or add two tablespoons of powder to 1 liter of water.
How to dilute concentrated detergent?
First, be sure to store your old laundry detergent bottles; when you open a new detergent bottle, pour half of the new detergent into the old empty bottle. Then take two bowls and fill them with water until about two-thirds separate them.
Conclusion
You can conclude from this article that laundry detergents have a more powerful cleansing effect than soap. Therefore, this property of detergents is used in our daily lives and industrial and commercial areas.